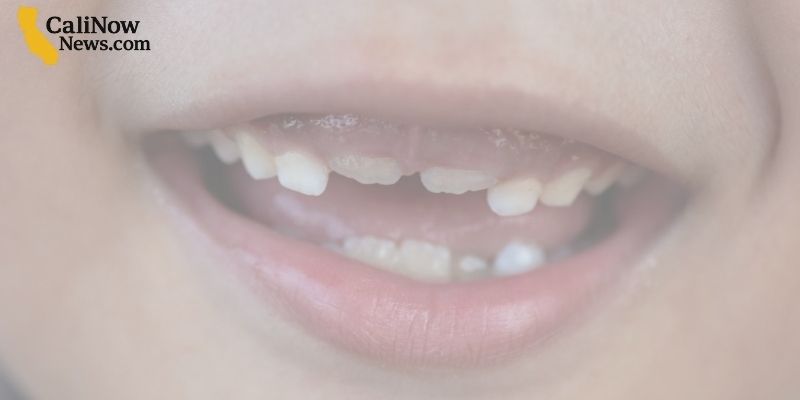
The U.S. Food and Drug Administration (FDA) has moved to ban over-the-counter fluoride supplements, citing concerns that they may harm the gut microbiome. However, many scientists argue that the evidence supporting this claim is limited and inconclusive.
Key Points:
- The FDA issued warnings to companies selling fluoride tablets and drops for children, stating they are “unapproved drugs” that could cause gut damage.
- The FDA’s action is based in part on a single 2023 study suggesting fluoride may alter gut bacteria in lab mice.
- Many dentists and medical researchers disagree, saying fluoride’s benefits for preventing cavities are well-established.
- Critics worry that this move could undermine public dental health, especially for low-income children who rely on supplements when fluoridated water isn’t available.
- The American Dental Association (ADA) has pushed back, defending the safety and efficacy of fluoride supplements.
Scientific Debate and Public Health Impact
What’s Behind the FDA’s Move?
The FDA recently warned several companies that their fluoride products are marketed without official approval, and cited potential gastrointestinal risks based on animal studies. However, the scientific consensus remains that such findings are not strong enough to justify pulling these products from the market.
Reactions from the Medical Community
Experts emphasize that dental cavities remain one of the most common childhood diseases, and fluoride—whether in water or supplements—plays a critical role in oral health. The ADA and pediatric groups stress that low-dose fluoride is both safe and effective.